What We Do
Alessa is at the forefront in developing new and innovative oncology strategies for treatment and prevention. We are actively developing implant and delivery systems and completing initial human feasibility testing. Our efforts are focusing on two of the most prevalent cancers that affect both men and women.
Prostate Cancer Overview

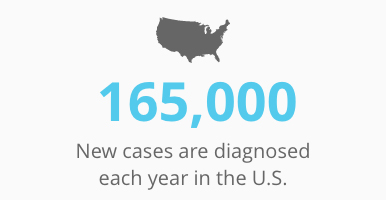
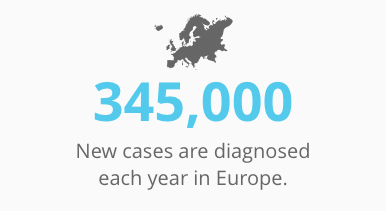
Some 1 in 6 men will be afflicted with prostate cancer during their lifetimes. About 165,000 new cases are diagnosed each year in the U.S., and 345,000 in Europe. Nearly 30,000 men die of this disease every year.
Current treatment options include either active surveillance (regular exams and biopsies) or whole-gland treatment (prostatectomy, which is removal of the prostate, or radiation therapy). However, these interventions have drawbacks. Active surveillance is insufficient for men with tumors of high metastatic potential. And whole gland treatment carries considerable risk of impotence and incontinence, and may be unnecessary in men whose tumors would not progress.
There is an urgent need for an intermediate treatment alternative that can reduce the risk of disease progression, but with fewer side effects than prostatectomy and radiation.

Breast Cancer Overview
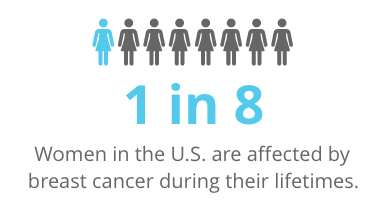

The most common invasive cancer in women, breast cancer will affect 1 in 8 women in the U.S. during their lifetimes. It is the second deadliest cancer for women, after lung cancer. In nearly all cases, it is caused by a genetic abnormality, either inherited or due to the aging process.
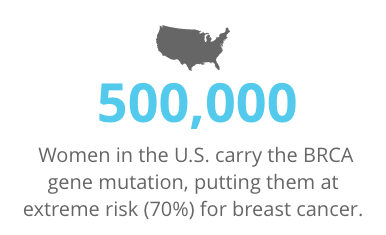
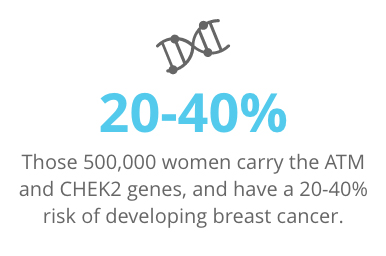
Approximately 500,000 women in the U.S. carry the BRCA gene mutation, putting them at extreme risk (70%) for breast cancer. The same number of women carry the ATM and CHEK2 genes, and have a 20-40% risk.
In addition, 60,000 women are diagnosed with DCIS non-invasive breast cancer, which can increase the risk of invasive cancer at a later date.
Clinical Trial

Alessa is currently conducting their first in-man clinical study of minimally invasive, implantable drug delivery to patients with prostate cancer. It would administer anti-androgen drugs to these patients through a sustained, localized system—without systemic exposure.
The primary objectives of the study are to assess the safety and patient tolerance of the product.
Alessa hopes to apply a similar technology for prevention and early intervention of breast cancer. It would involve implanting a reservoir of fulvestrant or similar drugs to breast tissue, where it would provide long-term, localized drug delivery.
To find out more information about our clinical study, visit ClinicalTrials.gov.
